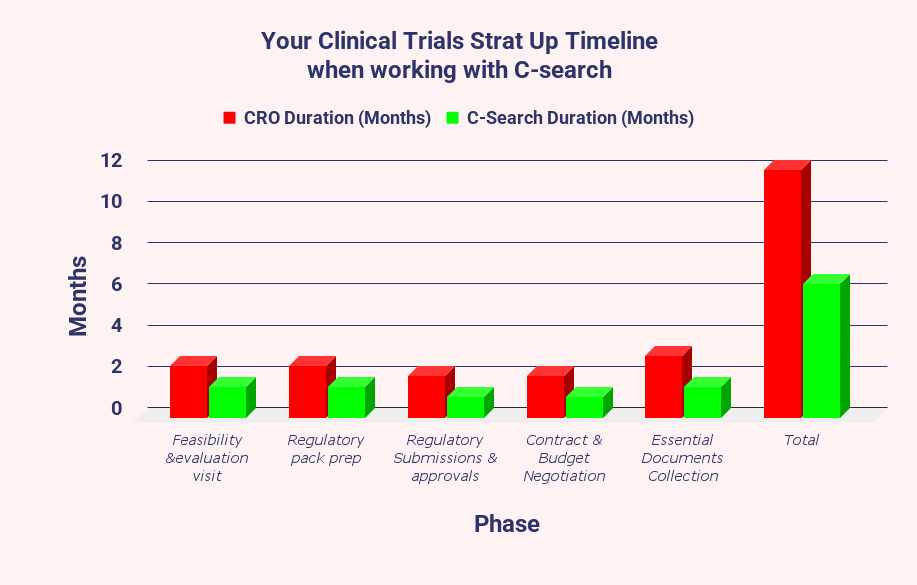
Our comparison underscores the efficiency of C-Search in the start-up phase of clinical trials. It typically achieves timelines that are approximately half of those of larger CROs.
This phase covers essential activities such as Feasibility Studies, Regulatory Submissions, the development of ICF and other essential documents, Contract and Budget Negotiations, and Collection of Essential Documents.
Learn more about working with IOMED
All rights reserved to C-Search LTD